PN-II-ID-PCE-2011-3-0856
Project title: IMMUNOMODULANTE FLUOROGLYCOPEPTIDE MOLECULAR
ARCHITECTURES
Project code: PN-II-ID-PCE-2011-3-0856
Project funding source: Executive
Agency for Higher Education, Research, Development and Innovation Funding
(UEFISCDI)
Contract number: 341/2011
Summary:
The project follows fundamental research activities that begin with the
elucidation and evaluation structure of new natural glycopeptides products, the
focus will be on medicinal plants of the Carpathian Mountains, the extracts of
which have already shown biological activities. In the area of marine natural
products research, compounds from marine invertebrates found in the Black Sea
will be explored. In particular the total synthesis and functionalization of
glycopeptides systems will be addressed. A key issue in natural products
research concerns the total synthesis of new immunomodulating
fluoroglycopeptides molecular architectures from natural products. Here, we
will develop new methodology enabling the functionalization of natural products
for photolabelling studies. By complex techniques and methodologies we will
investigate the partial and final structures of synthesized cromphore systems.
The design of compact tags unifiying fluorescent and photoreactive properties
will be a topic of basic chemical research as integral part of this project.
Project Scientific Objectives:
|
O.1 |
The
identification of new natural glycopeptides with biological activity and
selective human organ carrier properties from natural extracts isolated from
Carpathian flora and fauna of the Black Sea - through advanced basic research
to elucidate and evaluate these new bioactive structures as precursors for
new drugs. |
|
O.2 |
Structural
modification of natural glycopeptide systems selected from of natural
products by functionalization, and photolabelling of natural structure and
chemical synthesis of peptidomimetic analogues bioreactive with enhanced
properties. |
|
O.3 |
Synthesis and
characterization of new immunomodulating fluoroglycopeptides molecular
architectures using the photoreactive Ddz amino protected group Ddz by
innovative stereoselective total synthesis and biosynthesis methods of
peptide selected from the natural selected structures. |
|
O.4 |
Study on
molecular recognition processes on peptide-peptide and peptide-glycopeptide
interactions using amino acids or peptides as fluorescent linker, and
characterization of chromophore systems obtained. |
|
O.5 |
Testing the
biological activity of new immunomodulating fluoroglycopeptides molecular
architectures synthesized by computational chemistry and electrochemistry
techniques. |
|
O.6 |
Dissemination
of project results through web portal project, and participation to
international scientific and technical events in specific project areas, and
communicate scientific papers published in journals with internationally high
visibility, patent applications. |
Project team:
Dr. Ion NEDA, 64
years, organic chemist – Project Director
Dr. Ioan GROZESCU, 60 years - member
Dr. Adina-Elena SEGNEANU, 40 years - member
Dr. Paulina VLAZAN, 54 years- member
Dr. Raluca Oana POP, 32 years- member
Dr. Paula SFIRLOAGA, 37 years - member
Dr. Anamaria
DABICI, 28 years- member
Drd. Cristina MOSOARCA- member
Dr. Roxana BIRZOI, 33 years- member
Dr. Carmen LAZAU, 46 years- member
Dr. Corina ORHA, 35 years- member
Drd. Daniel URSU, 28 years- member
Dr. Cornelia BANDAS, 32 years- member
Valeria Nadina VLATANESCU, 30 years- member
Dr. Ionel BALCU, 44 years- member
Fedor Elena, 33 years- member
Maftei Catalin Vasile,33 years- member
Macarie Amalia Corina, 34 years- member
Martin Heiko Franz, 44 years- member
PROJECT BUDGET:
1,700,000 lei
PROJECT
DURATION: Oct. 2011 – Oct. 2015
SINGLE
PHASE 2011
- Identification of new bioactive peptide structures of plant extracts
The extraction
of biologically active compounds
Impetuous
development techniques for isolation and
identification of natural compounds resulted in immediate rapid achievement of
a wide range of compounds with special properties which have found use in various
areas important for mankind for isolation of natural product mixtures of interest
components are taken into account their
physical and chemical properties.
Extraction of
natural compounds from biological material (Artemisia annua, Anchusa
officinialis, Chelidonium majus, Eupatorium cannabinum, Helleborus spp., Viscum
album) are presented in the following stages:
-
Stabilization
and Drying
-
Grinding and
mixing
-
Extraction of
active compounds
Physico-chemical methods for
characterization of extracts by chromatography and
spectroscopy
Natural extracts
obtained from Artemisia annua plants, Anchusa officinialis, Chelidonium majus,
Eupatorium cannabinum, Helleborus spp., Viscum album and essential amino acids
were analyzed by standard methods: TLC (thin layer chromatography), HPLC (high
performance liquid chromatography) MS (mass spectrometry) and FT-IR (Fourier
transform infrared spectromerie). Before the characterization of the above
mentioned methods to chemically digested natural extracts in acid medium (HCl)
at a temperature of 1200C for 22 hours. The purpose of chemical
hydrolysis of peptide bonds was split into constituent aminoacids.
The
method TLC - For identification of peptidic
compounds in plant extracts above, initially developed RF identification
standard for essential amino acids, as well as plant extracts.

Photo image of the TLC plates for amino acids
Method
HPLC (high performance liquid chromatography)
- HPLC analysis of standard amino acids, saccharides standard and natural
extracts was performed with an HPLC chromatograph Dionex Ultimate 3000 detector
UVD-3000, Acclaim 120 C18 DIONEX, LPG pump-3400A.

HPLC Cromatrograma natural extract
hydrolyzed Anchusa officinialis
MS
method - Mass spectrometry experiments
were performed on an ion trap mass spectrometer
Ion Trap type Ultra High Capacity (HCT Ultra, PTM discovery) Bruker Daltonics
from. To prepare samples for analysis by mass spectrometry coupled with
NanoMate robot and achieve optimal experiments were used only reagents with
high purity and laboratory equipment and latest generation performance to meet
the needs of the study.

MS spectrum for the valine aminoacid
FT-IR
method - FT-IR analysis of standard
amino acids, saccharides standard and natural extracts was performed with a
Bruker Vertex 70 infrared spectrometric analysis in general, and its optimized
variant (Fourier transform) FTIR allows emphasizing "footprint" based
on specific functional groups, as well as structural changes that occur during
the chemical process. FTIR method allows qualitative and quantitative analysis
of the chemical composition of natural extracts and amino acids and sugars
standard.

Spectrul
FT-IR pentru extractul hidrolizat de
Anchusa officinialis
At
this stage, the following activities were carried out:
-
Preparing
for extraction of biological material: collection, drying, crushing, maceration.
-
Alcoholic
extract active compounds from plant material selected: Artemisia annua, Anchusa
officinialis, Chelidonium majus, Eupatorium cannabinum, Helleborus spp., Viscum
album.
-
Preparation
samples of vegetable extracts for identification and separation of the t amino
acids constituen by chemical hydrolysis;
-
Analysis
by chromatographic methods (TLC and HPLC) and spectroscopic (FT-IR and MS) of
amino acid standards.
-
Analysis by chromatographic methods (TLC and
HPLC) and spectroscopic (FT-IR) of saccharide standards
- Analysis and characterization of hydrolyzed alcoholic
extracts
SINGLE
PHASE 2012
Synthesis of new
derivatives based on the amino acids and monosaccharides
In the synthesis
of glycopeptides with biological activity, monosaccharides have a particularly
important role: they carry biological information selectively to cancer cells
affected organs. It is known that the structure of the sugar affect the
biological properties of peptides and proteins.
Given the
important role of amino acids in the body and monosaccharides of conveyors of
information.Biological were chosen for experiments of glucose and galactose
derivatives, and various amino acids.
2,3,4,6-Tetra-acetyl glucose is obtained by methods described in the literature, was used as starting material in condensation reactions with the protected amino acid to the amino group, to give the amino acid ester-glucopyranose. The condensation reaction takes place in the presence of two equivalents of imidazole, as a catalyst activator, and one equivalent of N, Ndiciclohexilcarbodiimida (DCC) with the purpose of activating the carboxyl group of the amino acid at 0 ° in dichloromethane.
Calixarene: separating agents
for natural amino
acid mixtures
Due to their molecular structure Calixarenele allows derivatization at
both phenolic OH group and in the para
position of the benzene ring, are selective extraction of metal ions and facilitate
the extraction of organic compounds from
mixtures or even the
separation of the enantiomers. Homocalix[3]arenas and derivatives of
calix[6]arene was synthesized in order to
use them as extracts of natural mixtures of
amino acids or active substances
in organic products, such as
3,4-di-Ocafeoilquinic
sunflower (product which inhibits the growth of cells
affected with the HIV virus). Acid calix[6]arene was
synthesized in two steps; in
the first step is
obtained hexaetil hexaacetic
acid ester 4-tert-butyl-calix[6]arene,
which is reduced to the acid by treatment with NaOH in
ethanol.
Surface
morphology analysis calixarene used to extract plant extract was performed by
scanning electron microscopy SEM and surface geometry calixarene by atomic
force microscopy (AFM).


The separation of peptides and amino
acids from plant extracts using solvents with different polarities,
We investigated a strategy of isolation amino acids and peptides
from natural extracts using solvents with different
polarities (CHCl3, CCl4, hexane, butanol, ethanol). To explain the chemical structure of the isolated compound was carried out by chromatographic techniques
(HPLC and GC-MS) and mass
spectroscopy. The results showed
that the fraction with hexane to extract
the highest number of amino acids, and ethanol were isolated fraction smallest
number of compounds.
Influence of
polarity chosen solvent in phase separation was investigated and evaluated by
GC-MS to isolate Thionins of Hellebore and Viscum, plants rich in
methionine. The obtained results have
confirmed that the low polarity solvents separate the largest number of amino
acids.
At this stage, the following
activities were carried out:
~
complete
synthesis and physico-chemical characterization of new derivatives of amino
acids and monosaccharides with a new groups of biologically active compunds in
the treatment of cancer (C). These results can be used in the step of amino
acid functionalizing or glycopeptide sequence, both compounds isolated from
natural products;
~ Cytotoxic and antiproliferative
activity testing in vitro of on 12 types of tumor cells ofor 5 substances synthesized N001-N005;
~ Studies on the synthesis and use as separating agents of Calixarenes,
selective extraction of amino acids, peptides and other small organic molecules
from natural mixtures - herbal extracts (hellebore and Viscum);
~
Identification
of biomolecules retained in calixarenes cavity by spectroscopic and
chromatographic techniques
~
Morphological and structural
comparative analysis of ligand by SEM and
AFM (calix acid [6]arena) and ligand-biomolecule;
~ The Development
new method for isolation (a series of
five different polar solvents) amino acids and peptides from natural extracts
and structural elucidation of separated
ompounds;
2013
Developing innovative new techniques in peptide synthesis architectures
by using the amino acid photoreactive stuck with photolabile groups (Ddz)
The
methods of peptide synthesis in solution of the natural peptides that generally
sensitive to acid or basic medium by the use of amino groups blocked by Fmoc
and Boc is made in low yields and formation of by-products of descopunere. This
is due to the fact that the breaking of the protecting group in acidic medium
is made.
Technicele new sensitive peptide synthesis in
solid phase (resin) using amino group stuck with α,
α-Dimethyl-3,5-dimethoxybenzyloxycarbonil (Ddz) eliminates these
deficiencies. Ddz is a blocking group for the amino acids that can replace Fotolia
Fmoc and Boc groups.
In Table 1
presents a synthesis technique peptide sequence H-Val-His-Leu-Tyr-Arg-Asn-Gly-Lys-OH
amino acids in the solid phase using Fmoc block groups and Ddz.
Table 1. The sequence of
H-Val-His-Leu-Tyr-Arg-Asn-Gly-Lys-OH
|
Scheme A resin: CTC-FmocLys loading: mmol/g |
Lot |
T0D0912 |
T0D0923 |
T0D0912 |
T0D1006 |
T0D1016 |
T0D1023 |
T0D1026 |
|
|
|
|
quantity: mmol |
Time/ min |
Ddz-Gly |
Ddz -Asn |
Ddz -Arg(Pbf) |
Fmoc-Tyr |
Fmoc-Leu |
Fmoc-His(1-Trt) |
Fmoc-Val |
Fmoc |
Ddz |
|
|
1
x DMF |
3 |
|
|
|
|
|
|
|
|
|
|
|
1
x 20% Piperidina/DMF |
7 |
|
|
|
|
|
|
|
X |
|
|
|
Iradiere |
Start |
28 h |
900 |
930 |
1005 |
|
|
|
|
X |
X |
|
end |
1300 |
1330 |
1305 |
|
|
|
|
|
X |
||
|
1
x 20% Piperidina/DMF |
Start |
25 |
|
|
|
1555 |
1135 |
1645 |
955 |
X |
|
|
end |
|
|
|
1620 |
1200 |
1710 |
1020 |
X |
|
||
|
1
x DMF |
|
|
|
|
|
|
|
|
|
|
|
|
2
x MeOH |
3 |
|
|
|
|
|
|
|
|
|
|
|
2
x DMF |
5 |
|
|
|
|
|
|
|
|
|
|
|
2
x MeOH |
3 |
|
|
|
|
|
|
|
|
|
|
|
2
x DMF |
5 |
|
|
|
|
|
|
|
|
|
|
|
Test
Ninhidrina |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
||
|
DDZ |
|
|
|
|
|
|
|
|
|
X |
|
|
Fmoc
acid |
|
|
|
|
|
|
|
|
X |
|
|
|
DIPCDI
[µL] |
|
|
|
|
|
|
|
|
X |
X |
|
|
HOBt
x H2O [g] |
|
|
|
|
|
|
|
|
X |
X |
|
|
TBTU
[g] |
|
|
|
|
|
|
|
|
X |
X |
|
|
DIPEA
[µL] |
|
|
|
|
|
|
|
|
X |
X |
|
|
Reaction medium |
|
|
|
|
|
|
|
|
X |
X |
|
|
beginning coupling |
|
|
|
|
|
|
|
|
X |
X |
|
|
beginning coupling |
|
|
|
|
|
|
|
|
X |
X |
|
|
end coupling |
|
|
|
|
|
|
|
|
X |
X |
|
|
1
x DMF |
3 |
|
|
|
|
|
|
|
X |
X |
|
|
2
x MeOH |
3 |
|
|
|
|
|
|
|
X |
X |
|
|
2
x DMF |
5 |
|
|
|
|
|
|
|
X |
X |
|
|
Ninhydrin Test |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
X |
X |
|
Synthesis of Novel Asymmetrically
Homotriazacalix[3]arene Derivatives and Their Extraction Behavior for Natural
Products
Elena Fodo, Catalin Vasile Maftei, Ion
Neda
A series of
homotriazacalix[3]arenes derivatives were synthesized for the extraction of
amino acids. The main driving force for the complexation was the interaction
between the ammonium cation of the amino acid and the oxygen atoms of the host
molecule. Stripping of amino acids was also accomplished by contacting the
organic solution with a fresh acidic solution. The homotriazacalix[3]arene
includes a guest molecule in the cavity, and the inclusion induces the
asymmetrization of the host molecule. This host compound functions as a
recognition tool for amino acids.
The ability of
calix[n]arenes to form complexes, to act as extractants in liquid–liquid
extraction, and run as carriers in transport through liquid membranes of
different biological amine derivatives (e.g., ammonium ion, amines, amino
acids, and peptides) has been the central topics of many reports. These
features recommend the homotriazacalix[3]arenes as compatible candidates for
studying the interactions involved in host–guest recognition as well as useful
receptors in separation processes. Some characteristic aspects of their
applications in binding and separation of various amine compounds by
extraction, and in transport through liquid membranes have therefore been
considered.

Synthesis of Novel Photoreactive
Peptides derivatives of 6-Methoxy Quinolinic Acid and Quinine
Catalin Vasile Maftei, Elena
Fodo, Ion Neda
Photoreactive peptides
are generated by their functionalization with
photo-active moieties and are used for observing bio-molecular interactions.
Using photo-conjugated methods that includes chemical or enzymatic degradation
processes, it can be identified the contact part of the molecule.
For the
generation of the photoreactive peptides with bioactivity witch can be used in
medicinal diagnostic or as transporters for already known bioactive compounds
(antitumor, bacteriostatic), we synthesized photo-active derivatives from natural products (from Quinine it was synthesized
6-Methoxy Quinolinic Acid) or we
used the chiral natural product itself Quinine. The coupling of the
photo-active moieties was made by modifying the amino function of
H-Val-His-Leu-Tyr-Arg-Asn-Gly-Lys-OH peptide in the α position or the free
hydroxyl groups.

Fotoreactivitate tests
were performed in UV and are shown in the pictures below


Helebrinei isolation of a
concentrated extract of Helleborus
We
tested several methods of isolating helebrinei of Helleborus. The best results
were obtained after selective extraction helebrinei with a mixture of
chlorinated hydrocarbons and alcohol. The roots of Helleborus defatted with
petroleum ether extracted with methanol in several stages. After evaporation of
the solvent of the concentrate to obtain a methanol extract reddish gum which
was treated with a mixture of distilled water and ethanol 10:1.
With a view to investigating the
interaction between the calixarene and helebrina and experiments were performed
using hexaacetic acid, p-tert-butyl-calix]6]arene and helebrina. There were
proton spectra for pure compounds and complex gained through interactions
between the two compounds.
New
Members of the Cinchona Alkaloid Family:
9-Aminoquincorine-10-aldehyde
and 9-Aminoquincoridine-10-aldehyde
Ion Neda,Elena Fodor,Catalin V. Maftei, Monica
Mihorianu,Horst-Dieter Ambrosi, and M. Heiko Franz Eur.
J. Org. Chem. 2013, 7876–7880
This study reports the synthesis of the new enantiopure
amino aldehydes, 9-aminoquincorine-10-aldehyde (1) and
9-aminoquincoridine-10-aldehyde (2). These alkaloid-like compounds are
derivatives of the Cinchona alkaloids quinine and quinidine. Their application
as chiral building blocks in the synthesis of novel compounds is demonstrated
by the reduction and reductive amidation of the aldehyde moiety. Furthermore,
their use in early drug discovery and supramolecular chemistry is described.
The synthesis of new chiral building blocks is of
general interest. 9-Aminoquincorine-10-aldehyde (1) and
9-aminoquincoridine-10-aldehyde (2) belong to the Cinchona alkaloid
family (Figure 1).

Figure 1. Chiral building
blocks: 9-aminoquincorine-10-aldehyde (1) and 9-aminoquincoridine-10-aldehyde (2).
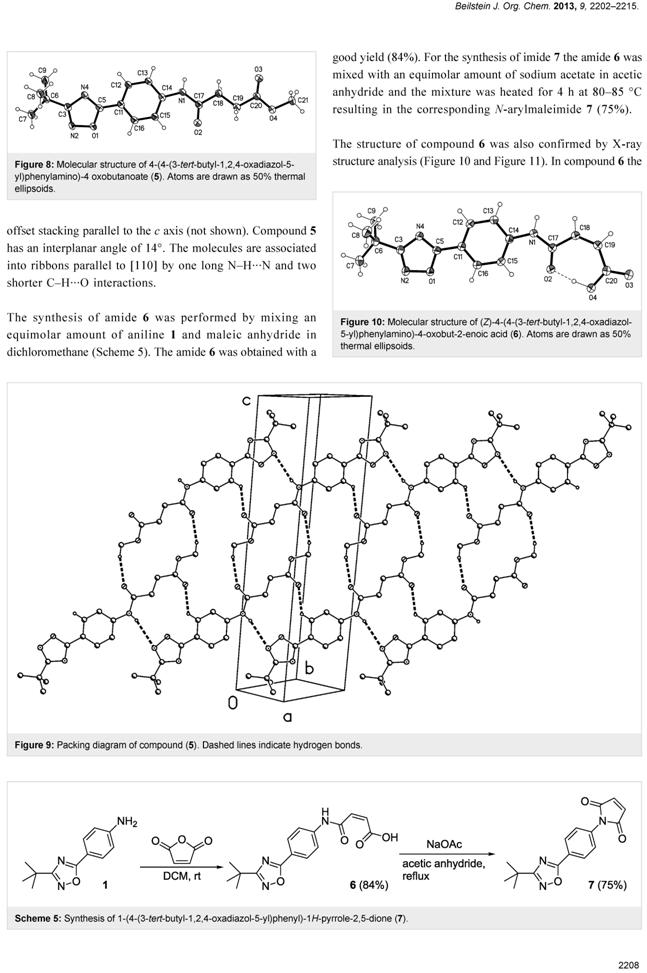
Synthesis and characterization of
novel bioactive 1,2,4-oxadiazole natural product analogs
bearing the N-phenylmaleimide
and N-phenylsuccinimide moieties
Catalin V. Maftei, Elena Fodor, Peter G. Jones1, M. Heiko Franz, Gerhard
Kelter, Heiner Fiebig and Ion Neda
Beilstein J. Org. Chem. 2013, 9, 2202–2215
Taking into consideration
the biological activity of the only natural products containing a 1,2,4-oxadiazole
ring in their structure (quisqualic acid and phidianidines A and B), the
natural product analogs
1-(4-(3-tert-butyl-1,2,4-oxadiazol-5-yl)phenyl)pyrrolidine-2,5-dione (4) and
1-(4-(3-tert-butyl-1,2,4-oxadiazol-5-yl)phenyl)-1H-pyrrole-2,5-dione (7) were
synthesized starting from 4-(3-tertbutyl-1,2,4-oxadiazol-5-yl)aniline (1) in
two steps by isolating the intermediates
4-(4-(3-tert-butyl-1,2,4-oxadiazol-5-yl)phenylamino)-4-oxobutanoic acid (3) and
(Z)-4-(4-(3-tert-butyl-1,2,4-oxadiazol-5-yl)phenylamino)-4-oxobut-2-enoic acid
(6). The two natural product analogs 4 and 7 were then tested for antitumor
activity toward a panel of 11 cell lines in vitro by using a monolayer cell-survival
and proliferation assay. Compound 7 was the most potent and exhibited a mean
IC50 value of approximately 9.4 μM.Aniline 1 was synthesized by two routes
in one-pot reactions starting from tert-butylamidoxime and 4-aminobenzoic acid
or4-nitrobenzonitrile. The structures of compounds 1, 2, 4, 5 and 6 were
confirmed by X-ray crystallography.
The five-membered
heterocyclic 1,2,4-oxadiazole motif is of synthetic and pharmacological
interest. It also forms an important constituent of biologically active
compounds including natural products [1]. Sawyer et al. have described such
compounds as bioisosteres for amides and esters, with the 1,2,4-oxadiazoles
showing higher hydrolytic and metabolic stability.


















SINGLE
PHASE 2014
Within
the framework of the project
"IDEE" a series of
modified calixarenes have been
synthesized which should be used in molecular
recognition. The nature of the modifications were in two directions. On one hand we introduced fluorescent groups
and on the other we coupled asymmetric
moieties, which based on the chiral
natural-product-based compounds QCI and
QCD. This modification and the ring size
affected the cavity
for molecular recognition of the Calixarens.
a) tetra-[Ph2P(O)-ethyl]-tert-butyl-calix[4]arene
b) 1,3-Di-[(6-Methoxy-chinolin-4-yl)carbonyl]-tert-butyl-calix[4]arene
c) 1,3-Di-{[(QCI-9-yl)carbonyl]methoxy}-tert-butyl-calix[4]arene
d)
tris-N-Benzyl-Homoaza-calix[3]aren
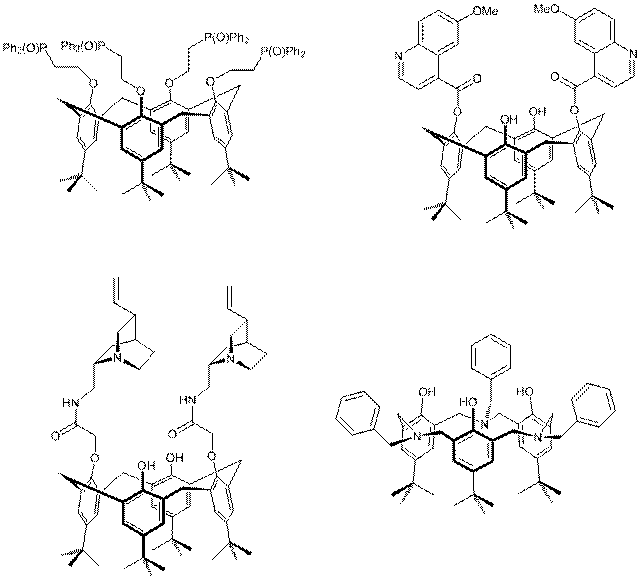
The
interaction of these molecules
with amino acids and peptides were
investigated by fluorescence-emission
spectroscopy and cyclic voltammetry.
An influence of the responds of the measurement were observed; especially
fluorescence-emission spectroscopy.
Novel Silver and Gold N-Heterocyclic Carbene Initiators in the
Ring- Opening Polymerization of ʟ-Lactide
C.
V. Maftei, E. Fodor, I. Neda
- 15th International Conference "
Polymers and Organic Chemistry", June 10-13, Timisoara, Romania
Because of their biodegradability,
biocompatibility and ready availability from inexpensive renewable resources, polylactides
(PLAs) are attracting growing interest as environmentally friendly substitutes
for petrochemical-based polymers. Accordingly, these synthetic polyesters have
given rise to a broad range of practical applications in packaging, surgery
(tissue and bone repairing and engineering), and pharmacology (controlled
release of active ingredients). PLAs are usually prepared by ring-opening
polymerization (ROP) of lactide via a coordination-insertion mechanism
involving a metal complex.
The growing utility of NHCs in
homogeneous catalysis is now well recognized and a multitude of NHCs catalysts
have been developed in recent years for a wide variety of transformations.
As the catalytic utility of
Au–NHC complexes remains largely unexplored and that of the Ag–NHC complexes is
just beginning to emerge, we became interested in the design of Au and Ag
complexes for their potential application in homogeneous catalysis. In
particular, we were interested in the design of Au–NHC and Ag–NHC based
initiators for the ring-opening polymerization (ROP) of ʟ-Lactide.
In summary, several new silver
and gold complexes supported over a difunctionalized N-heterocyclic
carbene ligand have been synthesized.
The gold and silver complexes
effectively catalyze the ROP of ʟ-Lactide
under solvent-free melt conditions to produce the polylactide polymer of
moderate to low molecular weight with a narrow molecular weight distribution.
Synthesis,
Design and Characterization of Gold (I) and Silver (I) NHC-complexes based on
imidazo[1,5-a]pyridine moiety for applications in the biomedical field
Monica Mihorianu, Ion Neda,
- 27-30 July, Prague, Czech Republic
Over the last decade Gold (I) and
Silver (I) N-heterocyclic carbene complexes have had an increasing impact in
different fields of applications as: catalysis , medicinal chemistry and are
still under research. Recent studies showed that silver(I) - NHC complexes have
in vitro anticancer activity against ovarian and breast cells3 and the gold(I)
NHC complexes inhibit the enzyme thioredoxin reductase, an enzyme relevant for
the proliferation of tumor tissue and its inhibition is related to the
triggering of anti-mitochondrial effects. This encourages further research on
N-heterocyclic carbenes-gold(I) and silver (I) complexes as antitumor agents.
We were interested in using the
imidazo[1,5-a ]pyridine moiety as a scaffold for new anticancer drugs, due to
the facts that imidazo[1,5-a]pyridine core is easily accessible4, their salts
proves to be valuable antimicrobial agents5, and form very stable metal
complexes. In particular, we were interested in the design of Au-NHC and Ag-NHC
complexes based on imidazo[1,5-]pyridine- 3-ylidenes for applications in the
biomedical field.
Synthesis, characterization and biological
studies of two new series of unsymmetrical substituted NHC gold(I) and
silver(I) complexes derived from imidazo[1,5-a]pyridine-3-ylidenes were
investigated. Using Paclitaxel as a standard, all Au(I) and Ag(I)-NHC complexes
were evaluated for their in vitro antitumor activity against 11 and, 12 cell
lines respectively by using a monolayer cell survival and proliferation assay
Gold(I) Alkynyl
Complexes Derivatives of Cinchona Alkaloids. Synthesis and Antitumor Activity
E.
Fodor, Catalin Maftei, Ion Neda - 15th
International Conference " Polymers and Organic Chemistry", June
10-13, Timisoara, Romania
There
is continued interest in the chemistry of alkynyl gold(I) complexes primarily
resulting from their physical properties, such as, luminescence, non-linear
optical behaviour and liquid crystalline properties. Like platinum(II), gold(I)
is well-known to form metal-metal interactions in solution and the solid state.
These aurophilic interactions can give rise to photophysical properties such as
luminescence. Interest in these phenomena has led to the development of a range
of gold(I)-containing materials. One class of complexes that has been
extensively studied in this regard (because they tend to form robust air and
water-stable organometallic compounds) are the gold(I) alkynyl complexes. The
gold(I) alkyne fragment is most often found complexed to phosphine, carbene, or
isonitrile two-electron-donor ligands, forming neutral linear complexes.
However, the steric bulk associated with the phosphine and carbene ligands can
prevent close contacts between adjacent Au(I) ions.
Cinchona
alkaloids have been proved to be efficient antimalarial and antibacterial drug
candidates. It is well-documented that the application of quinine derivatives
in the field of cancer detection and in chemotherapy goes far back to the past.
One of the most widely used
methods of preparing new (alkynyl)gold(I) compounds centres on the
depolymerization reaction of neutral homoleptic alkynylgold(I) polymers, upon
addition of good σ-donor ligands such as phosphines, isocyanides or
halides. Such reactions have allowed the synthesis of a huge number of new
neutral and ionic gold(I) alkynyl complexes.
We assume that alkynylgold(I) polymers adopt
structures similar to other gold complexes of this type, whereby each gold atom
is assumed to be simultaneously σ-coordinated to one alkynyl fragment and
π-bonded to the carbon–carbon triple bond of an adjacent molecule, but
cannot rule out the possibility of -NH2 or -OH coordination. In summary,
several new cinchona alkaloids gold(I) complexes have been synthesized and
tested for in vitro anti-tumor activity towards a panel of 11 cell lines
using a monolayer cell survival and proliferation assay.
QCI-NH2-Au-PPh3 and QCD-NH2-Au-PPh3 were found to have a good tumor selectivity.
Bifunctional
Derivatives Of The Isophosphoramide Mustard
Birzoi R., Kelter G. , Fiebig H.
, Neda I. - 20th International
Conference on Organic Synthesis, 29 June-4 July 2014 - Budapest, Hungary
Although, novel alkylating agents for cancer therapy have
been developed during the last decade, e.g. palifosfamide1 and
glufosfamide2, there is still unmet medical need for more effective
and better tolerated anticancer drugs. The importance of amino acids and
glycosides in the metabolism, as well as the role as selective carriers of the
biological information played by monossacharides have already been established.
Taking into consideration these facts, we focused on the
development of bifunctional compounds that will combine the selective
transporting properties of monossacharides with the active metabolite
isophosphoramide mustard. We have also synthesized fluoroglycopeptides that can
be used in the study of cell interactions in order to highlight the biological
activity of the synthesized compounds..
Sugar derivatives, amino acids or monosaccharide–amino acid
moieties were conjugated with the N,N'-Bis(2-chloroethyl)phosphorodiamidic
acid to give the corresponding salts.

The in vitro antiproliferative/cytostatic activity of some
synthesized compounds was tested on eleven tumor cell lines. Despite the low
stability at ambient temperature – decomposition was observed by 31P-NMR, some
derivatives are stable at temperatures below –15°C for limited periods of time.
Synthesis of Novel
Homotriazacalix[3]arene Derivatives and Their Extraction Behavior E.Fodro, C.V. Maftei, I
Neda,- Tagung, GDCH,06.05.-08.05.2014 Braunschweig, Germania
The ability of
calix[n]arenes to form complexes, to act as extractants in liquid– liquid
extraction, and run as carriers in transport through liquid membranes of
different biological amine compounds (e.g., ammonium ion, amines, amino acids,
and peptides) has been the central topics of many reports. These features
recommend the homotriazacalix[3]arenes as compatible candidates for studying
the interactions involved in host–guest recognition as well as useful receptors
in separation processes. Some characteristic aspects of their applications in
binding and separation of various amine compounds by extraction, and in
transport through liquid membranes have therefore been considered.
The homotriazacalix arenes
includes a guest molecule in the cavity, and the inclusion induces the
asymmetrization of the host molecule. This host compound functions as a
recognition tool for amino acids.
A series of homotriazacalix
arenes derivatives were synthesized for the extraction of amino acids. The main
driving force for the complexation was the interaction between the ammonium
cation of the amino acid and the oxygen atoms of the host molecule. Stripping of
amino acids was also accomplished by contacting the organic solution with a
fresh acidic solution. The homotriazacalix arene includes a guest molecule in
the cavity, and the inclusion induces the asymmetrization of the host molecule.
This host compound functions as a recognition tool for amino acids
A mixture of
homotriazacalix[3]arenes, potassium carbonate and ethyl bromoacetate in dry
acetone was stirred and heated under reflux for 24 h. The cooled solution was
filtered through a bed of Celite and the filtrate was concentrated to dryness.
A mixture of the triester and 15% aq. sodium hydroxide in ethanol was stirred
and heated under reflux for 24 h after which most of the ethanol was distilled
off. The residue was diluted with cold water and HCl was added with vigorous
mixing until pH 1 was reached. The solid was then filtered off and dried.
The
experimental results suggested that amino acid methylesters are extracted from
aqueous phase into organic phase at acidic pH values. The extractability was
proved to be essentially controlled by the structure of calix[n]arene and the
nature of the amino acid. The results suggested further possibilities for
optimal separation of amino acids derivatives and other biological species by
means of derivative calixarenes.
Synthesis of Novel
Photoreactive Peptides derivatives of 6- Methoxy Quinolinic Acid and Quinine,
C.
V. Maftei, E. Fodor, I. Neda
- Tagung, GDCH,06.05.-08.05.2014 Braunschweig, Germania
Photoreactive
peptides are generated by their functionalization with photo-active moieties
and are used for observing bio-molecular interactions. Using photo-conjugated
methods that includes chemical or enzymatic degradation processes it can be
identified the contact part of the molecule.
For the generation of
the photoreactive peptides with bioactivity witch can be used in medicinal
diagnostic or as transporters for already known bioactive compounds (antitumor,
bacteriostatic), we synthesized photo-active derivatives from natural products
(from Quinine it was synthesized 6-Methoxy Quinolinic Acid) or we used the
chiral natural product itself Quinine.
The coupling of the photo-active moieties was
made by modifying the amino function of H-Val-His-Leu-Tyr-Arg-Asn-Gly-Lys-OH
peptide in α position or the free hydroxyl groups.
Novel photoreactive peptides were synthsised
by coupling H-Val-His-Leu-Tyr-Arg-Asn- Gly-Lys-OH with chiral natural product
Quinine and 6-Methoxy Quinolinic Acid derivate from Quinine using different
activating agents for the acidic function. The use of 2-Cl-1- Methyl Pyridinium
Iodide is selective for the generation of the esters. The DCC coupling is not
selective and the activated acid is coupled both to the OH and NH2 affording
the corresponding esters and amides.
Isolation by affinity
chromatography of the bioactive products from helleborus purpurascens and investigation of their anti-tumor
activity towards a panel of 12 cell lines
Birzoi R., Balcu I., Macarie C., Kelter G
and Neda I- 30-31 oct.2014 Bucuresti Priorităţile chimiei pentru o dezvoltare
durabilă - PRIOCHEM
Further
investigation will focus on the biological, especially antitumoral activity of the helleborus extracts and isolated
derivatives as hellebrine, glycosides and peptide. In vitro
anti-tumor activity of 9 extracts
and hellebrine towards a panel of
12 cell lines was assessed using a monolayer cell survival and proliferation
assay. By exhibiting a mean IC50 value of 0,007 µg/ml fraction
6 was the most potent fraction (peptide fraction) followed by hellebrine (mean
IC50 = 0.011 µg/ml. Individual IC50 values of
sample 6 were in the range from 0.003 µg/ml (lung cancer cell line LXFL
529) and 0,012 µg/ml (ovarian cancer cell line OVXF 899), corresponding to
a 7.2-fold difference between the most sensitive and most resistant cell line.
Individual IC50 values of hellebrine were in the range from
0.003 µg/ml (lung cancer cell line LXFL 529) and 0.029 µg/ml (ovarian
cancer cell line OVXF 899. Individual IC50 values of sample 9 were
in the range from 0.005 µg/ml (lung cancer cell line LXFL 529) and
0,038 µg/ml (ovarian cancer cell line OVXF 899).
Accepted papers
Catalin V.
MAFTEI, Elena FODOR, Peter
G. JONES, Constantin G. DANILIUC, M.
Heiko FRANZ, Gerhard KELTER, Heiner FIEBIG,
Matthias TAMM and Ion NEDA “Novel Bioactive 1,2,4-Oxadiazole Natural Product
Analogs; Synthesis, Structural Analysis And Potential Antitumor Activity” Received November 19, 2014
Catalin V. Maftei, Elena
Fodor, Peter G. Jones, Matthias Freytag,M. Heiko Franz, Corneliu M. Davidescu and Ion Neda:“Asymmetric Calixarene Derivatives as Potential Hosts
in Chiral Recognition Processes”
SINGLE
PHASE 2015









Disemination:
Peptide and
Amino Acids Separation and Identification from Natural Products by I. Neda, P. Vlazan, R.O. Pop, P.Sfarloaga, I.
Grozescu, A.E. Segneanu, in the book - Analytical Chemistry, Ed. by Ira S. Krull, ISBN 978-953-51-0837-5, Publisher: InTech, November
07, 2012, p.135-146.
Synthesis and characterization of novel bioactive 1,2,4-oxadiazole natural
product analogs bearing the N-phenylmaleimide and N-phenylsuccinimide moieties
by C. V. Maftei, E. Fodor, P. G. Jones, M. H. Franz, G.
Kelter, H. Fiebig and I. Neda, Beilstein J. Org. Chem. 2013, 9, 2202-2215
New Members of the Cinchona Alkaloid Family: 9-Aminoquincorine-10-aldehyde
and 9-Aminoquincoridine-10-aldehyde by I. Neda, E. Fodor, C. V. Maftei, M. Mihorianu, H. D. Ambrosi and M. H.
Franz, Eur. J. Org. Chem. 2013, 35, 7876.
The Influence of Extraction Process Parameters of Some Biomaterials
Precursors from
Helianthus annuus by A. Segneanu, I. Grozescu, P. Sfirloaga, Digest Journal of Nanomaterials and
Biostructures Vol. 8 (4), p. 1423- 1433 (2013)
National and International Conferences
1. A. E. Segneanu, I. Grozescu, C. Lazau, C. Bandas, A.
Dabici, N. Vlatanescu, I. Neda, Characterization of some important natural compounds
from Cheledonium major, International Conference Chimia 2012 “New Trends in Applied Chemistry“, May
24 – 26, 2012, Constanta, Romania, PA1.
2. Grozescu, A. E. Segneanu, C. Lazau, C. Bandas, A.
Dabici, N. Vlatanescu, I. Neda, Isolation and analysis of some important natural
compounds from Anchusa officinalis International Conference Chimia 2012 “New Trends in Applied Chemistry“,
May 24 – 26, 2012, Constanta, Romania, PA10. I.
3. Segneanu A.E., Grozescu Ioan, Sfirloaga Paula, Pop
Raluca, Neda Ion,Studies On Peptides and Glycopeptides from Viscum Sp.,
13th Congress of the International Society for
Ethnopharmacology, Graz, Austria, September 2 - 6, 2012, P426.
4. I. Grozescu, A.E. Segneanu, P. Sfirloaga, A. Dabici,
I. Neda, Physico-Chemical Characterization of Biological Active Compounds from
Helianthus Annuus, 6th
Interntional Conference on Materials Science and Condensed Matter Physics
(MSCMP 2012), Chisinau, Moldova, CPPP 39 P;
5. A.E. Segneanu, I.Grozescu, P.Sfirloaga, R.Pop, I.Neda,
Studies on Chemical Composition of Helleborus puspurascens, XXXII-nd Romanian
Chemistry Conference, Oct. 2012, Calimanesti-Caciulata, Valcea, Romania,
P.S.I.22;
6. A.E. Segneanu, I. Grozescu, P. Sfirloaga, R. Pop, P.
Vlazan, I. Neda, Studies on Peptides and Free Amino Acids from Eupatoria
cannabium, Simpozionul
internaţional „Priorităţile Chimiei Pentru o Dezvoltare
Durabilă Priochem” ediţia a VIII-a, 25 - 26 octombrie 2012, Bucureşti, Romania
7. A.-E. SEGNEANU, P. SVERA, M. CHIRITA, L. KOSS, P.
Sfirloaga, I. GROZESCU, Synthesis of a Natural Cyclic Peptide from Hellebore
sp. as Biomaterial Precursors, 4rd International Conference "Research
People and Actual Tasks on Multidisciplinary Sciences", 12– 16 June
2013, Lozenec, Bulgaria.
8. A.-E. SEGNEANU, P. VLAZAN, A. PETRIC, P. Svera, I.
Grozescu, Magnetic Cobalt Ferrite Nanoparticles: Synthesis and Surface
Functionalization with Peptide, BRAMAT 2013, International Conference On
Materials Science & Engineering, 28 February – 2 March 2013, Braşov,
Romania.
9. P. Sfirloaga, A. Segneanu, P. Vlazan, A. Dabici, I.
Neda, I. Grozescu The influence of extraction method on isolation of biological
active compounds from sunflower (Helianthus annuus), XVII International Sol-Gel Conference, August 25-30, 2013 in Madrid, Spain.
10.
Ion Neda, Heiko Franz Small bio-active molecules with potential cytostatic activity Summer School, july 8-10, 2013, Petru Poni Institute of Macromolecular
Chemistry, Iasi, Romania
11. Claudia Lar, Anamaria Terec, Ion Neda, Ion Grosu Synthesis and Structural Analysis of
Some New Hexahomotrioxacalix[3]arene Derivatives Based on Terpyridine Units Frühjahrssymposium 2013, in Berlin, Jung Chemiker Forum , Berlin, 06.03. 2013
12. Segneanu, P. Svera, I. Grozescu Peptide with Potential Biological Activity Simpozionul International PRIOCHEM, editia a IX-a, 24-25 octombrie 2013
Conferinte Plenare 2014
Universitatea Politehnica Timişoara şi Societatea de Chimie din
România, Filiala Timişoara, a organizat o conferință pe teme
actuale din domeniul chimiei susţinută de Prof.dr. Ion NEDA de la
Universitatea Tehnică din Braunschweig, Germania.
Manifestarea a avut loc miercuri, 29 ianuarie 2014, ora 11:00, în Sala
Senatului Universităţii Politehnica Timişoara.
Synthesis of
asymmetric Calixarene-derivatives as potential hosts in chiral recognition
procrsses, 15th International Conference " Polymers and Organic Chemistry",
June 10-13, Timisoara, Romania Heiko Franz, Corneliu-Mircea Davidescu, Ion
Neda,
Postere
2014
1. C. V. Maftei, E. Fodor, I. Neda Synthesis of Novel Photoreactive Peptides derivatives of 6-Methoxy
Quinolinic Acid and Quinine, Tagung, GDCH, Braunschweig, 06.05-08.05. 2014
2. E. Fodor, C.V. Maftei, I. Neda Synthesis of Novel Asymmetrically Homotriazacalix[3]arene Derivatives
and Their Extraction Behavior for Natural Productes,Tagung, GDCH,
Braunschweig, 06.05-08.05. 2014
3. C. V. Maftei, E. Fodor, I. Neda Novel Silver and Gold N-Hetrocyclic Carbene
Initiators in the Ring-Opening Polymerization of L-Lactide, 15th
International Conference " Polymers and Organic Chemistry", June
10-13, Timisoara, Romania
4. E. Fodor, Catalin Maftei, Ion Neda Gold (I) Alkynyl Complexes Derivatives of
Cinchona Alkaloids. Synthesis and
Antitumor Activity, 15th International Conference " Polymers and
Organic Chemistry", June 10-13, Timisoara, Romania
5. Birzoi R. Kelter G. Fiebig H. Neda I. BIFUNCTIONAL
DERIVATIVES OF THE ISOPHOSPHORAMIDE MUSTARD, 20th International Conference
on Organic Synthesis, 29 June-4 July 2014 - Budapest, Hungary
6. Monica Mihorianu, Ion Neda, Synthesis, Design and Characterization of Gold (I) and Silver (I)
NHC-complexes based on imidazo[1,5-a]pyridine moiety for applications in the
biomedical field, Chirality, 27-30 July, Prague, Czech Republic
7. Birzoi R. Balcu I, Macarie C, Kelter G
and Neda I. Isolation by Affinity Chromatography of the Bioactive
Products from Helleborus Purpurascens and Investigation of their Anti-Tumor
Activity Towards A Panel Of 12 Cell Lines PRIOCHEM 30-31 oct 2014 Bucuresti
SINGLE
PHASE 2015
PROJECT PLANNING
|
Budget Year |
Type of stage |
Stage number * |
Budget for each stage |
Date of beginning and |
|
2011 |
Single stage |
1 |
193.401,00 |
05/10/2011 - 15/12/2011 |
|
2012 |
Single stage |
2 |
548.188,00 |
16/12/2011 - 16/12/2012 |
|
2013 |
Single stage |
3 |
243.643,88 |
17/12/2012 - 18/12/2013 |
|
2014 |
Single stage |
4 |
209.384,00 |
19/12/2013 - 16/12/2014 |
|
2015 |
Single stage |
5 |
201.063,00 |
17/12/2014 - 31/12/2015 |
|
2016 |
Single stage |
6 |
304.320,12 |
01/01/2016 - 03/10/2016 |
PLAN DE REALIZARE A
PROIECTULUI
|
Year |
Stage |
Objectives |
Activities |
Results per phase |
|
2013 |
Single stage |
Synthesis of new biologically active fluoropeptides |
Innovative methods of stereo selective
total synthesis of biologically active fluoro peptide architecture from
natural peptide structure selected from natural products |
Scientific papers communicated and
published in high impact international journals, stage report |
|
Physical and chemical
characterization of intermediate and
final compounds |
||||
|
2014 |
Single stage |
Studies of molecular recognition
processes or protein-protein interactions by using fluorescent amino acids as
linkers. Fluorescent glycopeptides, necessary in the recognition processes,
synthesis |
Labeling of natural structure and
chemical synthesis of biologically reactive peptidomimetic analogs with
improved properties |
Scientific papers communicated and
published in high impact international journals, stage report |
|
Utilization of fluorescent amino
acids or peptides in molecular recognition processes or protein-protein
interactions |
||||
|
Separation and identification of
biologically active fractions from Helleborus. Contributions for developing a
new “Drug-Targeting” concept for treating cancer |
Synthesis of calixarene and –resorcinarene
of variable cavity and their use in
selective extractions experiments |
|||
|
Biologic activity testing |
Physical and chemical
characterization (NRM) the intermediate and final compounds |
|||
|
2015 |
Single stage |
Specific interactions of
biologically active compounds |
NMR studies of association
complexes (identified structures) |
Scientific papers communicated and
published in high impact international journals, stage report |
|
Biologic activity testing |
Voltametric and clinical methods for characterizing
synthesized chromophore systems. |
|||
|
clinical voltammetry studies -
stage 2 (for studies of molecular recognition of nonfotoreactive peptides
using photoreactive calixarene) |
||||
|
2016 |
Single stage |
Studies of molecular recognition
processes by using fluorescent glycopeptides as linkers. |
Studies of the relationship
between chemical structure and biological activity of the synthesized
glycopeptide system |
Scientific papers communicated and
published in high impact international journals, stage report |
|
Biologic activity testing |
Clinical voltammetry studies and
spectrochemical methods for synthesized systems chromophore (further on). |
Posters
2015
Plenary
conference
1. „Control of Structure and Functionality of Amino
Acids and Natural-Based Glycopeptides by Incorporating them in Host Molecules”susţinută de Prof. Dr. Ion Neda
Conferinaţa „19 th Romanian International Conference on Chemistry and
Chemical Enginering” 2-5 septembrie 2015 Sibiu
Scientific
papers published – ISI
SINGLE PHASE 2016
PROJECT PLANNING
|
Budget Year |
Type of stage |
Stage number * |
Budget for each stage |
Date of beginning and |
|
2011 |
Single stage |
1 |
193.401,00 |
05/10/2011 - 15/12/2011 |
|
2012 |
Single stage |
2 |
548.188,00 |
16/12/2011 - 16/12/2012 |
|
2013 |
Single stage |
3 |
243.643,88 |
17/12/2012 - 18/12/2013 |
|
2014 |
Single stage |
4 |
209.384,00 |
19/12/2013 - 16/12/2014 |
|
2015 |
Single stage |
5 |
201.063,00 |
17/12/2014 - 31/12/2015 |
|
2016 |
Single stage |
6 |
304.320,12 |
01/01/2016 - 03/10/2016 |
PLAN DE REALIZARE A
PROIECTULUI
|
Year |
Stage |
Objectives |
Activities |
Results per phase |
|
2013 |
Single stage |
Synthesis of new biologically active fluoropeptides |
Innovative methods of stereo
selective total synthesis of biologically active fluoro peptide architecture
from natural peptide structure selected from
natural products |
Scientific papers communicated and
published in high impact international journals, stage report |
|
Physical
and chemical characterization of
intermediate and final compounds |
||||
|
2014 |
Single stage |
Studies of molecular recognition
processes or protein-protein interactions by using fluorescent amino acids as
linkers. Fluorescent glycopeptides,
necessary in the recognition processes, synthesis |
Labeling of natural structure and
chemical synthesis of biologically reactive peptidomimetic analogs with
improved properties |
Scientific papers communicated and
published in high impact international journals, stage report |
|
Utilization
of fluorescent amino acids or peptides in molecular recognition processes or
protein-protein interactions |
||||
|
Separation
and identification of biologically active fractions from Helleborus. Contributions
for developing a new “Drug-Targeting” concept for treating cancer |
Synthesis
of calixarene and –resorcinarene of
variable cavity and their use in selective extractions experiments |
|||
|
Biologic activity testing |
Physical
and chemical characterization (NRM) the intermediate and final compounds |
|||
|
2015 |
Single stage |
Specific interactions of
biologically active compounds |
NMR studies of association
complexes (identified structures) |
Scientific papers communicated and
published in high impact international journals, stage report |
|
Biologic activity testing |
Voltametric and clinical methods for characterizing
synthesized chromophore systems. |
|||
|
clinical
voltammetry studies - stage 2 (for studies of molecular recognition of
nonfotoreactive peptides using photoreactive calixarene) |
||||
|
2016 |
Single stage |
Studies of molecular recognition
processes by using fluorescent glycopeptides as linkers. |
Studies of the relationship
between chemical structure and biological activity of the synthesized
glycopeptide system |
Scientific papers communicated and
published in high impact international journals, stage report |
|
Biologic activity testing |
Clinical voltammetry
studies and spectrochemical methods for synthesized systems chromophore (further on). |
Studies
developed in the project in 2016
1. It was
tried different methods of extraction, separation and identification of the
free amino acids or of other biological compounds from the hydro-ethanolic
extract of Helleborus or from the aqueous extract obtained from the roots of Helleborus,
products obtained by INCEMC Timisoara from the firma "Exhelios" din Timisoara.
2. The
extractions from the aqueous solution with n-butanol,
respectively the analytical methods, leaded to the identification and isolation
in pure form of the steroid "Hellebrine" using affinity
chromatography. There were also identified other derivatives of the steroid,
Dracoside and another compound that has a hexose group in minus.
3. It was
performed several synthesis of new products which represents the main core of
some natural products isolated from marine species. The cytostatic activity of
the new synthesized products and their Au(I) and Ag(I) complexes was studied at
the German firma ONCOTEST on 12 types of human cancer cells. The tested
compounds exhibit remarkable cytostatic activity.
4. It was
synthesized a series of peptides contained by the Thionines identified in the
Helleborous delivered by the firma EXHELIOS: Tetrapeptide, Hexapeptide and
Octapeptide.
5. A series
of amino acids and peptides were labeled with an aziridine compound and
photo-activated by irradiation at 350 nm (the synthetic methods and the
photo-activation are described in detail).
Studies of
molecular recognition processes by using fluorescent glycopeptides as linkers.
The idea of photoaffinity labelling is that that a
small molecule carrying a photophore binds to a macromolecule, e.g. peptide.
After the small photophore coordinates to the contact side the solution is
irradiated and the photophore generates a covalent bond. After cleavage of the
macromolecule the unit can by identified at which photophore coordinates.

[3-(Trifluoromethyl)-3H-diazirin-3-yl]arenes
were introduced as photophores by Brunner et al. [2] to provide a more
favourable carbene than the one produced from diazoesters or from
aryldiazirines.
Lindel et al.
investigated the chemical selectivity [3]. They found that for Tyr-Val-dipeptide
the O-benzylation of the phenol-moiety is favoured against C-benzylation
of it or other benzylations.

Site-specific incorporation of
[3-(Trifluoromethyl)-3H-diazirin-3-yl]arenes into peptide:

Fragments identification:

Results:
The peptide linked covalent to the
photophore was cleaved in the single amino acids and the supposed amino acids
linked to the photophore. This mixture was analysed by mass spectroscopy
(electro spray; ESI-MS). The calculated masses for the amino acids linked to
the former diazirin are given in the table.
|
Fragment |
Calculated Mass |
Found Mass |
Intensity |
|
|
[Dalton] |
|
|
|
Val-Diazirine |
275,1133 |
276,1302 |
trace |
|
His-Diazirine |
313, 1038 |
314,1162 |
micro |
|
Leu- Diazirine |
289,1290 |
290,1311 |
trace |
|
Tyr- Diazirine |
339,1282 |
340,1293 |
major |
|
Arg- Diazirine |
332,1460 |
333,1521 |
trace |
|
Ala- Diazirine |
247,0820 |
- |
n.d. |
|
Gly- Diazirine |
233,0664 |
- |
n.d. |
|
Lys- Diazirine |
304,1399 |
305,1432 |
trace |
Posters
2016
Plenary
conference
1. Ion
NEDA “New
Targets for Quincorine and Quincoridine Cinchona Alkaloid based key structures „ Modern
Biotechnologies in Sustainable Development of the Danube Delta May 31-June 2,
2016
2. Ion Neda, Catalin V. Maftei, Elena Maftei3, M. Heiko Franz “Natural product based
Heterocycles and Peptides with Cytostatic Activity” New trends and Strategies in the chemistry of advanced materials
with relevance in biological systems, technique and environmental protection
June 09-10, 2016 Timisoara
3. Ion
Neda, Catalin V. Maftei, Elena Fodor, M. Heiko Franz „Control Of Structure And Functionality Incorporating Reactive Species
In A Calixarene Cavity:Development Of New Synthetic Methods For Innovative Drug
Design” a XXXIV-a Conferinta Nationala de
Chimie 04 – 07 octombrie, 2016 Călimăneşti –
Căciulata, judeţul Vâlcea, ROMÂNIA
4. M.
Heiko Franz, Catalin V. Maftei, Elena Fodor, Ion Neda “Cinchona Alkaloid Based Key Structures:
Quincorine And Quincoridine And Derivatives Thereof”
a XXXIV-a
Conferinta Nationala de Chimie 04 – 07 octombrie, 2016 Călimăneşti –
Căciulata, judeţul Vâlcea, ROMÂNIA
Scientific
papers published – ISI